 Most carbon in the soil is lost as greenhouse gas (carbon dioxide, CO2) into the atmosphere if natural ecosystems are converted to agricultural land. Soils contain 3.3 times more carbon than the atmosphere and 4.5 times more than plants and animals on earth (1). This makes soils an important source of greenhouse gases but also a potential sink if right management is applied. The use of crop residues for bio-energy production reduces the carbon stocks in cropland. Further the dedication of cropland to bio-fuel production increases the area of cultivated land and thus carbon loss from soils and vegetation.
Most carbon in the soil is lost as greenhouse gas (carbon dioxide, CO2) into the atmosphere if natural ecosystems are converted to agricultural land. Soils contain 3.3 times more carbon than the atmosphere and 4.5 times more than plants and animals on earth (1). This makes soils an important source of greenhouse gases but also a potential sink if right management is applied. The use of crop residues for bio-energy production reduces the carbon stocks in cropland. Further the dedication of cropland to bio-fuel production increases the area of cultivated land and thus carbon loss from soils and vegetation.Pyrolysis of waste biomass can generate fuels and biochar recalcitrant against decomposition. If biochar is returned to agricultural land it can increase the soil’s carbon content permanently and would establish a carbon sink for atmospheric CO2. In this case the use of crop residues as a potential energy source may improve soil quality and reduce greenhouse gas emissions in a complementary not competing way. Biochar is proposed as a soil amendment in environments with low carbon sequestration capacity and previously depleted soils (especially in the Tropics). From previous studies it is known that soil biochar amendments increase and maintain soil fertility (2) and the human-made Terra Preta soils in the Ama-zon prove that infertile soils can be transformed into fertile soils and long term carbon enrichment is feasible even in environments with low carbon sequestration capacity (3).
Read more about the global carbon cycle, climate change, soil organic carbon and our options and prospects to mange this carbon pool by biochar carbon sequestration.
The Greenhouse Effect and Climate Change
Climate change and global warming are two terms used for the predicted and observed increase in temperature. While the current temperature increase is caused by human influence on the earth’s carbon cycle, the greenhouse effect is a naturally occurring process. In fact, without this process life on planet Earth would be rather unlikely. Short-waved radiation from the sun is able to permeate the atmosphere (about 55%). The reflected radiation from Earth’s surface has a longer wave length (infrared). The majority of this outgoing radiation is absorbed by the so called greenhouse gases (such as carbon dioxide, water vapor, methane, and nitrous oxide) in the atmosphere (Figure 1).
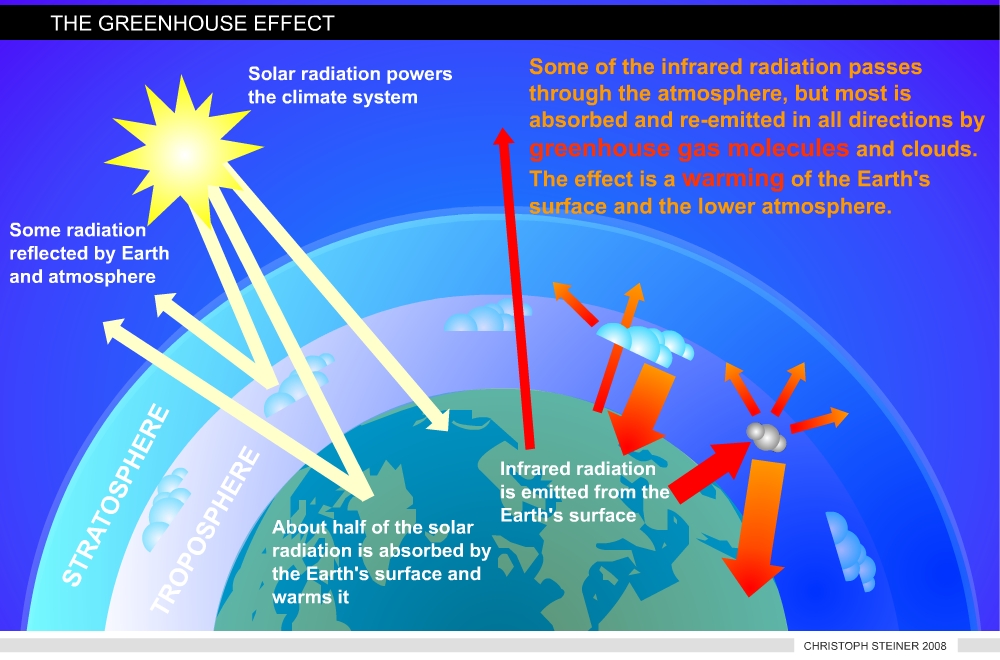 |
| Figure 1 illustrates the Greenhouse Effect (redrawn from www.ipcc.ch) |
This process changes the energy balance of the planet just as the glass roof of a greenhouse. This natural process rises the Earth’s temperature by 33° Celsius to an average of 15° Celsius. The amount of heat energy retained by the atmosphere is controlled by the concentration of greenhouse gases and they are balanced by the action of life. Without life the composition of the Earth’s atmosphere would be different.
Read more about The Carbon Cycle
The global carbon cycle exchanges carbon (as CO2) between carbon reservoirs. These include (4): • The atmosphere (720 Gt = billion tons) • The terrestrial biosphere (2,000 Gt) • the oceans (38,400 Gt) • marine sediments and rocks (Lithosphere > 60,000,000 Gt) and fossil fuels (4,130 Gt) = coal (3,510 Gt), oil (230 Gt), gas (140 Gt) and others (250 Gt)
Terrestrial ecosystems and the oceans exchange CO2 rapidly with the atmosphere. The carbon exchange from the lithosphere is very slow, although some CO2 is released by volcanoes. CO2 is removed from the atmosphere through photosynthesis and stored in organic matter. When plants grow they utilize sunlight, CO2 and water (H2O) to synthesize organic matter (photosynthesis) and release oxygen (O2, see equation 1).
Equation 1 Light
↓
CO2 + 6H2O → C6H12O6 + 6O2
This organic matter is returned to the atmosphere by decomposition of dead plant tissue or distur-bances, such as fire, in which large amounts of organic matter are oxidized and rapidly transferred into CO2. Terrestrial carbon is primarily stored in forests (5). In undisturbed full-grown forest ecosystems, the turnover time of carbon is on the order of decades and uptake by photosynthesis and release by decay is balanced.
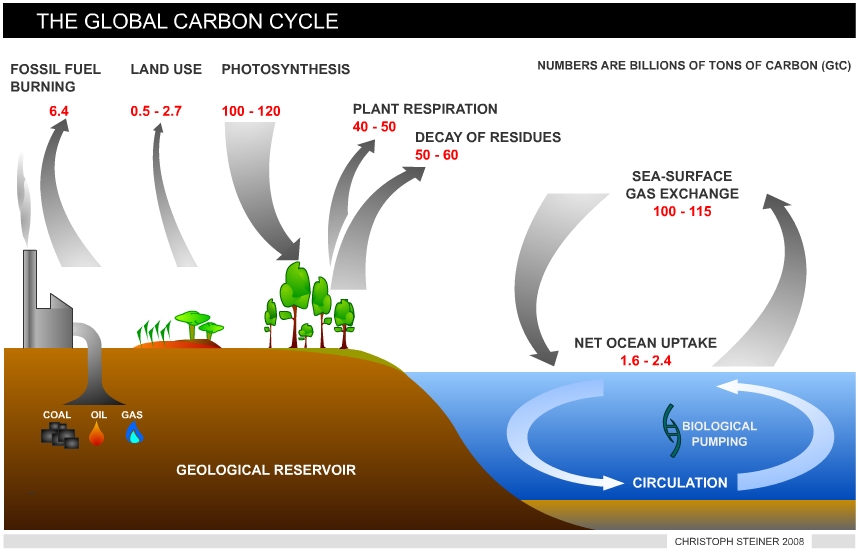 |
| Figure 2 Sources and sinks of CO2 - The global carbon cycle |
Read more about the Human influence on the carbon cycle and greenhouse gases
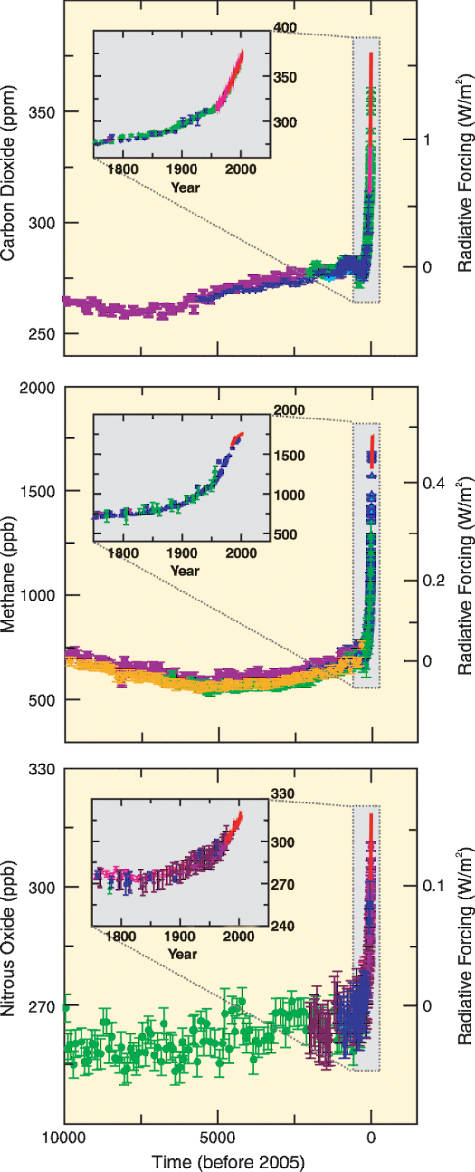 |
Atmospheric CO2 increased to 379ppm in 2005 (Figure 3). Ice core
records reveal that we have left the domain that defined the Earth
system for the 420,000 years before the Industrial Revolution (4) in a
speed never occurred before (Figure 3). CO2 is the most important anthropogenic GHG and its annual emissions grew by about 80% between 1970 and 2004. The current level exceeds by far the natural range over the last 650,000 years. The in-creases in GHG concentrations are mainly caused by fossil fuel burning and land-use change provides another significant contribution (Figure 5) Greenhouse Gas (GHG) Emissions from Agriculture Measurable anomalous emissions of GHG began already 8000 years ago. These early anthropogenic CO2 emissions were caused by forest clearing in Eurasia for agricultural purposes, and methane (CH4) emission rose from widespread rice irrigation about 5000 years ago (6). After 1750 the increase in atmospheric CO2 was mainly caused by fossil fuel combustion but emissions from land use change contributed about 30%, from which more than half is estimated from depletion of carbon in the soil. This depletion is exacerbated by further soil degradation and desertification (7). The total soil carbon (organic and inorganic) is 3.3 times the size of the atmospheric carbon pool (1). As most agricultural soils have lost 50 to 70% of their original carbon (7) they represent a considerable carbon sink if efforts are made to restore soil organic carbon, but also a huge source of GHG if soil management and deforestation rates are not changed. There is high agreement and much evidence that with current climate change mitigation policies and related sustainable development practices, global GHG emissions will continue to grow over the next few decades (25-90% between 2000 and 2030) (8). 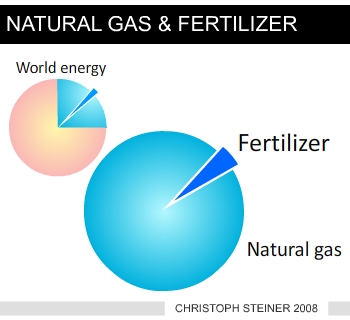 |
| Figure 3 increases in the most important greenhouse gases (www.ipcc.ch) | Figure 4 use of fossil energy (nat-ural gas) for nitrogen (fertilizer) synthesis |
Read about The Importance of Soil Organic Carbon
Soil organic carbon is not only an important source or sink of CO2 but also important for soil fertility. Before the invention of mineral fertilizers, management of organic carbon was the only way to restore or maintain soil fertility. Sedentary farmers either depleted their carbon stocks for nutrients, facing nutrient depletion, or found ways to maintain soil organic carbon. Migration is the solution to nutrient depletion for an estimated 300 to 500 million people affecting al-most one third of the planet’s 1500 million ha of arable land (9, 10). This agricultural system is termed “shifting cultivation”, indicating moving from one spot to another as soil fertility declines. Decreasing soil carbon contents correlate with a decline in agricultural productivity. The relationship between soil fertility and soil organic carbon was well known in the first half of the 19th century as the German agronomist Albrecht Thaer published his “Humus Theory”. Thaer’s approach, and quantitative assessment of agro-ecological and economic sustainability of farming systems was used with success during half a century, until 1849 when Sprengel and Liebig published on mineral nutrition of plants (13). From then on the “minimal nutrition theory” progressively abandoned recycling of nutrients from settlements to agricultural fields (14). Mineral fertilization boosted crop production and replenished nutrient stocks but did not treat soil degradation accompanied by accelerated loss of carbon. The observed loss of soil organic carbon is associated with yield decreases, reduced nutrient cycling and reduced nutrient-use efficiency of applied fertilizer (3, 9, 11, 12).
 |
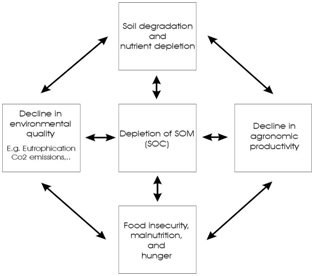 |
| Figure 6 Manmade Terra Preta soil in the Brazilian Amazon. These soils were enriched with charcoal and nutrients and prove that long lasting carbon enrichment and sustained soil fertility is possible even in the tropics. | Figure 7 shows the values of soil organic matter (SOM) and its implications on the environment, agronomy, and quality of life. Redrawn and lightly modified from (Lal 2004) |
Throughout the world intensive agricultural land use often has resulted in soil physical and chemical degradation, erosion, and higher losses than input rates of nutrients and organic materials. In contrast, the intentional and unintentional deposition of nutrient-rich materials within human habitation sites and field areas has in many cases produced conditions of heightened fertility status. An anthropogeni-cally-enriched dark soil found throughout the lowland portion of the Amazon Basin and termed Terra Preta de Índio is one such example (16). These soils contain high concentrations of charcoal (17); signifi cantly more plant available nutrients than in the surrounding soils (18). The existence of Terra Preta proves that infertile soils can be transformed into permanently fertile soils in spite of rates of weather-ing 100 times greater than those found in the mid-latitudes. Such a transformation cannot be achieved solely by replenishing the mineral nutrient supply (3).
It is important to separate effects due to organic matter per se (maintenance and improvement of water infiltration, water holding capacity, structure stability, retention of nutrients, healthy soil biologi-cal activity) from those due to decomposition (19, source of nutrients). Carbon is an important indicator of soil quality, and has numerous direct and indirect impacts on it such as, improved structure and tilth, reduced erosion, increased plant-available water capacity, water purification, increased soil biodiversity, improved yields, and climate moderation. This is essential to sustain the quality and productivity of soils around the globe, particularly in the tropics where there is a greater proportion of nutrient poor soils with a greater susceptibility to carbon loss (Due to faster decomposition in a hot and humid climate).
Read about The Biochar Approach
Increasing carbon stocks in soils with conventional means e.g. conservation tillage, use of manures, and compost, conversion of monoculture to complex diverse cropping systems, meadow-based rotations and winter cover crops, and establishing perennial vegetation on contours and steep slopes can sequester carbon. The sequestration potential depends on climate, soil type, and site specific management. The drawback of carbon enrichment with conventional methods is that carbon level drops rapidly again, as soon as the required careful management is no longer sustained. Carbon contents of cropland increases only if either carbon additions (in form of plant biomass) are enhanced or decomposition rates reduced (20). Only one-third of the aboveground residues remain in the soil after 1year and only 10-20% remains after 2 years. Furthermore the addition of degradable crop residues and reduced tillage systems can increase nitrous oxide and methane (N2O and CH4, both potent GHGs) emissions substantially.
 |
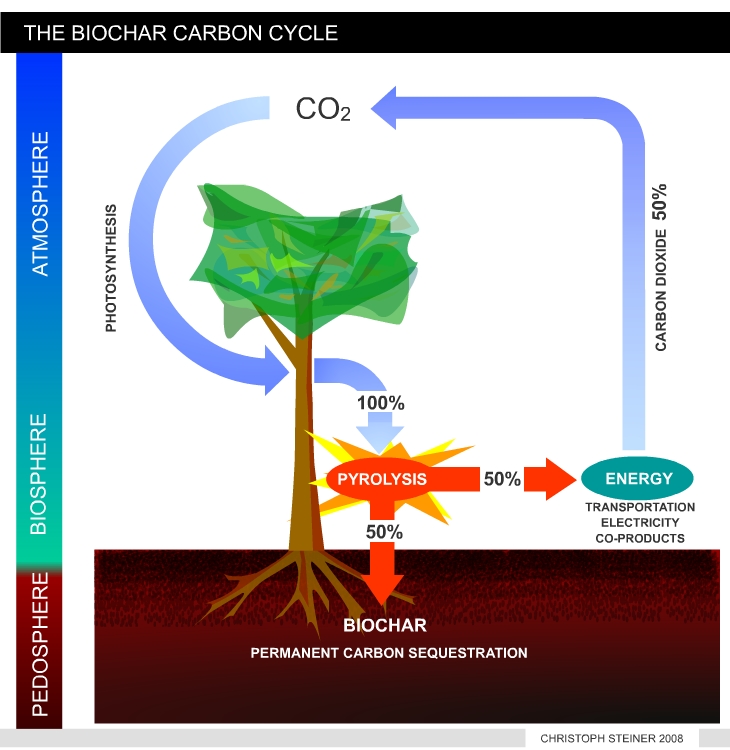 |
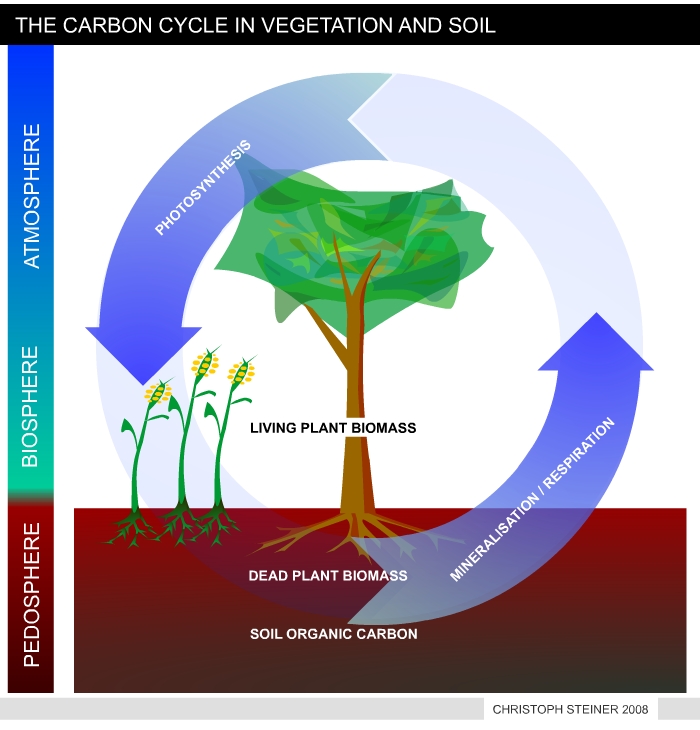
| Figure 8 shows a simplified version of the carbon cycle in vegetation and soil. Plants take CO2 from the atmosphere to synthesize tissue (plant biomass). As long as biomass is growing it accumulates carbon. During decomposition of dead biomass and humus the carbon is released as CO2. In undisturbed ecosystems the accumulation and release of CO2 is in equilibrium. | Figure 9 illustrates the manipulated carbon cycle due to bio-char carbon sequestration. Biochar is recalcitrant against decomposition and remains in the soil for centuries or mil-lennia. Thus pyrolysis can transfer 50% of the carbon stored in plant tissue from the active to an inactive carbon pool. The remaining 50% of carbon can be used to produces energy and fuels. This enables carbon negative energy generation if re-growing resources are used. (I.e. with each unit if energy produced CO2 is removed from the atmosphere). |
 |
Reduced decomposition is an advantage of biochar (Figure 10). Biochar
formation has important impli-cations for the global carbon cycle. In
natural and agroecosystems residual charcoal is produced by in-complete
burning. As the the soil carbon pool declines due to cultivation, the
more resistant charcoal fraction increases as a portion of the total
carbon pool (21-23) and may constitute up to 35% of the total (23).
Carbon dating of charcoal has shown some to be over 1500 years old,
fairly stable, and a permanent form of carbon sequestration (7).
Inspired by recreation of Terra Preta, slash and char was described as
an alternative to slash and burn (24). If a forest is burned, only
around 2-3% of the above-ground carbon is converted into charcoal (25),
but charcoal production can capture 50% of the above-ground carbon. If
re-growing resources (fallow vegetation or crop residues) are used,
slash and char could become a significant carbon sink and an important
step towards sustainability of tropical land use systems.
The global potential of biochar reaches far beyond slash and char.
Systems (pyrolysis) converting bio-mass into energy (hydrogen-rich gas
and bio-oil) and producing biochar as a by-product offer an opportunity
to combine renewable energy production, carbon sequestration and soil
restoration. Biochar can be produced by incomplete combustion from any
biomass, and it is a by-product of the pyrolysis technology used for
biofuel and bioenergy production.
The carbon cycling from photosynthesis and decomposing organic
materials is 50 to 60 billion tons (Gt) per year and land use emits
approximately 0.5 to 2.7 Gt of carbon (Figure 2). It would make a
significant global impact, if only a small fraction of this carbon flux
is altered by biochar carbon sequestration. Carbonization of
agricultural and forestry wastes could capture 0.16 Gt carbon yr-1. If
the demand for renewable fuels by the year 2100 was met through
pyrolysis, biochar sequestration could exceed current emissions from
fossil fuels (26). |
| Figure 10 demonstrates the historical knowledge about the recalcitrance of charcoal. Wooden poles were (are) blackened (carbonized) on the outside to increase their persistence in soil (photo C. Steiner). |
Read about Biochar and Soil Fertility
The recalcitrant nature of charcoal makes biochar rather exceptional. Whether biochar can improve soil quality to the same extent as decomposable organic materials is a valid question. Recent studies showed that soil biochar amendments are indeed capable of increasing soil fertility by improving chemical, biological, and physical properties. Biochar significantly increase plant growth (2, 27) and improve the efficiency of nitrogen fertilizers (27, 28).
 |
| Figure 11 shows sorghum plants growing on mineral fertilized (NPK) soil. The plot to the left got additional biochar amend-ment (11 tons per ha). (Embrapa Research Station, Manaus, Brazil, Photo C. Steiner) |
This has important consequences as the synthesis of nitrogen fertilizers consumes large amounts of fossil energy (Figure 3) and the cost of nitrogen fertilizer increases dramatically with increasing energy prices. The effects on soil biology seem to be essential as biochar has the potential to alter the microbial biomass (29) and composition (30) and the microbes are able to change the biochar’s properties (17). The majority of experiments conducted show that biochar soil amendments result in enhancement of beneficial fungi (31) and nitrogen fixing microbes (32). The improved productivity ranges from 20 to 220% at application rates of 0.4 to 8 tons carbon ha-1 (33).
Read about Advantages of Biochar Carbon Sequestration
• No competition between SOC restoration, bio-fuels and food production
Numerous researchers warn of deleterious effects on soil fertility if crop residues are removed for bio-energy production (34-39). Removing more than 25% of cornstalks reduced productivity (38).
Pyrolysis with biochar carbon sequestration provides a tool to combine sustainable soil management (carbon sequestration), and renewable energy production. While producing renewable energy from biomass, carbon sequestration, agricultural productivity, and environmental quality can be sustained and improved if the biomass is transferred to an inactive carbon pool and redistributed to agricultural fields. The uses of crop residues as potential energy source or to sequester carbon and improve soil quality can be complementary, not competing uses.
The global amount of crop residue produced is estimated at 2.8 Gt per year for cereal crops and 3.8 Gt for 27 food crops. The use of crop residues for bioenergy could save 10-25% of fossil energy use (20).
An enormous amount of biomass is burned each year without any use. Frequently biomass (forests, fallow vegetation, grassland, crop residues) is burned to get rid of it, adding CO2 to the atmosphere for only marginal and short term increases in soil fertility. Burning of biomass (slash and burn, crop residue burning) is a common practice, releases nearly all the carbon stored in the biomass immediately as CO2 and barely adds to carbon buildup in agricultural soils.
Many agricultural systems search for alternatives to crop residue burning, because accumulating resi-dues can cause considerable crop management problems. E.g. increasing residue incorporation in flooded rice paddies causes higher CH4 emission, a very potent greenhouse gas. Other residues increase the risk of diseases and insect attack.
Worldwide, the total carbon release from fire is of the order of 4-7 Gt of carbon per year. This flux is almost as large as the rate of fossil fuel consumption (Figure 2) (40). Tropical forest conversion is esti-mated to contribute globally as much as 25 % of the net CO2 emissions (Palm, et al., 2004). These num-bers emphasize the potential for biochar carbon management if only the biomass is utilized that is burnt for no other use than getting rid of it.
• Pyrolysis or gasification with biochar carbon sequestration
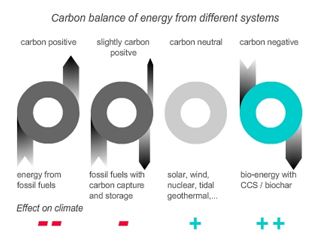 |
Carbon capture and storage (CCS) usually assumes geo-sequestration (CO2 capture in depleted oil and gas fields, saline aquifers etc.) as the sequestering tool. To capture and store carbon as CO2 is very cost-intensive. These technologies require vast capital inputs and large scale projects. Using this technology for coal power plants can at best reduce its CO2 emissions, while using re-growing biomass would establish a carbon sink. Bio-energy with biochar carbon sequestration would capture a maximum of 50% of the carbon stored in the biomass but offers several environmental advantages. Bioenergy with biochar carbon storage facilitates the generation of carbon-negative energy (Figure 9 and 12). |
| Figure 12 shows the carbon foodprint of different energy systems. Only bio-energy with carbon capture and storage or bio-char carbon sequestration can produce carbon negative energy i.e. if re-growing biomass is used, this system reduces CO2 from the atmosphere with each unit of energy produced. (www.biopact.com). | |
Biochar producing gasifiers can have a broad range in size and in technological complexity. Bio-char can be produced as a byproduct from cooking (biochar producing kitchen stoves, Ropert Flanagan, personal communication, Figure 13). Decentralized small scale projects are feasible and large capital investments are not necessary. As biochar is a byproduct of gasification, no carbon capture technology is necessary. There is no risk of harmful CO2 leakage from biochar. Most scientists agree that the half life of biochar is in the range of centuries or millennia.
 |
 |
 |
| Figure 13 shows a biochar producing cooking stove. Left picture shows carbonized corncobs remaining in the stove after gasification. (R. Flanagan, www.charion.co.uk) | ||
• Fast SOC buildup beyond the maximum sequestration capacity
From biomass to humus a considerable fraction of carbon is lost by respiratory processes, and also from humus to resistant soil carbon. Only 2-20% of the carbon added as above ground residues and root biomass enters the soil organic carbon pool by humification. The rest is converted to CO2 due to oxidation, and furthermore humus is not inert to oxidation (1). Soils can only sequester additional carbon until the maximum soil carbon capacity, or soil carbon saturation, is achieved, which requires a steady input of biomass and careful management practices. 80-90% of the carbon from crop residues in the field is lost due to decomposition in the first 5 to 10 years. In contrast, about 50% of the carbon can be captured if biomass is converted to biochar (26).
The existence of Terra Preta proves that SOC enrichment beyond the maximum capacity is possible if done with a recalcitrant form of carbon such as biochar. These soils still contain large amounts of bio-char derived SOC in a climate favorable for decomposition, hundreds and thousands of years after they were abandoned.
• Reduced deforestation
The carbon trading market holds the prospect to reduce or eliminate deforestation of primary forest, because cutting intact primary forest would reduce the farmer’s carbon credits. (41) estimated the above-ground biomass of unlogged forests to be 434 tons per hectare, about half of which is carbon. This carbon is lost if burned in a slash and burn scenario and lost to a high percentage (> 50%) if used for biochar production. Therefore, only re-growing plant biomass can establish a carbon sink. The carbon trade could provide an incentive to cease further deforestation; instead reforestation and recuperation of degraded land for fuel and food crops would gain magnitude. As tropical forests account for between 20 and 25% of the world terrestrial carbon reservoir (42), this would reduce emissions from tropical forest conversion which is estimated to contribute globally as much as 25 % of net CO2 emissions and up to 10 % of N2O emissions to the atmosphere (43).
A prerequisite for the above mentioned management practices is access to the global carbon trade. According to (36) the global C market has a potential grow to $1 trillion by 2020 or before. This market must be made accessible to land managers, especially in the tropics where sustaining soil organic carbon and soil fertility is most challenging and CO2 emissions due to land use change are highest. One tone of biochar is roughly the equivalent of 3 tons CO2.
Cited Literature
1. R. Lal, Science 304, 1623 (2004).
2. C. Steiner et al., Plant and Soil 291, 275 (2007).
3. W. Zech, L. Haumaier, R. Hempfling, in Humic substances in soil and crop sciences; selected readings, P. McCarthy, C. E. Clapp, R. L. Malcolm, P. R. Bloom, Eds. (American Society of Agronomy and Soil Science Society of America, Madi-son, 1990), pp. 187-202.
4. P. Falkowski et al., Science 290, 291 (2000).
5. R. K. Dixon et al., Science 263, 185 (1994).
6. W. F. Ruddiman, Climatic Change 61, 261 (2003).
7. R. Lal, Critical Reviews in Plant Sciences 22, 151 (2003).
8. IPCC, “Fourth Assessment Report, Climate Change 2007: Synthesis Report” (IPCC, 2007).
9. J. G. Goldammer, in Fire in the Environment: The Ecological Atmospheric, and Climatic Importance of Vegetation Fires, P. J. Crutzen, J. G. Goldammer, Eds. (John Wiley & Sons Ltd, 1993), pp. 297-314.
10. C. P. Giardina, R. L. Sanford, I. C. Dockersmith, V. J. Jaramillo, Plant and Soil 220, 247 (2000).
11. D. Hölscher, B. Ludwig, R. F. Möller, H. Fölster, Agriculture Ecosystems & Environment 66, 153 (1997).
12. M. C. Silva-Forsberg, P. M. Fearnside, in Management and Rehabilitation of Degraded Lands and Secondary Forests in Amazonia, J. A. Parrotta, M. Kanashiro, Eds. (International Institute of Tropical Forestry, Rio Piedras, 1995), pp. 90-95.
13. C. L. Feller, L. J.-M. Thuriés, R. J. Manlay, P. Robin, E. Frossard, J. Plant Nutr. Soil Sci. 166, 687 (2003).
14. R. J. Manlay, C. Feller, M. J. Swift, Agriculture Ecosystems & Environment 119, 217 (2007).
15. R. Lal, R. F. Follett, B. A. Stewart, J. M. Kimble, Soil Science 172, 943 (2007).
16. W. I. Woods, in Papers and Proceedings of the Applied Geography Conferences, F. A. Schoolmaster, Ed. (Applied Geo-graphy Conferences, Denton, Texas, 1995), vol. 18, pp. 158-165.
17. B. Glaser, G. Guggenberger, L. Haumaier, W. Zech, in Sustainable management of soil organic matter, R. M. Rees, B. C. Ball, C. D. Campbell, C. A. Watson, Eds. (CABI Publishing, Wallingford, 2001), pp. 190-194.
18. H. N. Lima, C. E. R. Schaefer, J. W. V. Mello, R. J. Gilkes, J. C. Ker, Geoderma 110, 1 (2002).
19. W. G. Sombroek, F. O. Nachtergaele, A. Hebel, Ambio 22, 417 (Nov, 1993).
20. D. R. Sauerbeck, Nutrient Cycling in Agroecosystems 60, 253 (2001).
21. W. Zech, G. Guggenberger, in Humic substances in terrestrial ecosystems, A. Piccolo, Ed. (Elsevier, 1996).
22. J. Skjemstad, paper presented at the Net Ecosystem Exchange Workshop Proceedings, Canberra ACT 2601, Australia, 18-20 April 2001 2001.
23. J. O. Skjemstad, D. C. Reicosky, A. R. Wilts, J. A. McGowan, Soil Science Society of America Journal 66, 1249 (Jul-Aug, 2002).
24. C. Steiner, W. G. Teixeira, W. Zech, in Amazonian Dark Earths: Explorations in Space and Time, B. Glaser, W. I. Woods, Eds. (Springer Verlag, Heidelberg, 2004), pp. 183-193.
25. P. M. Fearnside, P. M. Lima, A. Graça, F. J. A. Rodrigues, Forest Ecology and Management 146, 115 (2001).
26. J. Lehmann, J. Gaunt, M. Rondon, Mitigation and Adaptation Strategies for Global Change 11, 403 (2006).
27. J. Lehmann et al., Plant and Soil 249, 343 (2003).
28. C. Steiner et al., Journal of Plant Nutrition and Soil Science, (2008).
29. C. Steiner, W. G. Teixeira, J. Lehmann, W. Zech, in Amazonian Dark Earths: Explorations in Space and Time, B. Glaser, W. I. Woods, Eds. (Springer Verlag, Heidelberg, 2004), pp. 195-212.
30. J. J. Birk, Diplomarbeit, University of Bayreuth (2005).
31. D. D. Warnock, J. Lehmann, T. W. Kuyper, M. C. Rillig, Plant and Soil 300, 9 (2007).
32. M. A. Rondon, J. Lehmann, J. Ramirez, M. Hurtado, Biology and Fertility of Soils DOI 10.1007/s00374-006-0152-z, (2007).
33. J. Lehmann, M. Rondon, in Biological Approaches to Sustainable Soil Systems, N. U. e. al., Ed. (CRC Press, Boca Raton, FL, USA, 2006), pp. 517-530.
34. R. Lal, Environment International 31, 575 (2005).
35. R. Lal, Soil & Tillage Research 96, 1 (2007).
36. R. Lal, Soil Sci Soc Am J 71, 1425 (2007).
37. R. Lal, D. Pimentel, Soil & Tillage Research 93, 237 (2007).
38. H. Blanco-Canqui, R. Lal, Geoderma 141, 355 (2007).
39. D. R. Sauerbeck, Nutrient Cycling in Agroecosystems 60, 253 (2001).
40. J. Goudriaan, in Carbon sequestration in the biosphere. Processes and prospects., M. A. Beran, Ed. (Springer Verlag, Heidelberg, Berlin, 1995), vol. 33, pp. 3-18.
41. P. M. Fearnside, Climatic Change 35, 321 (1997).
42. M. Bernoux et al., in The biogeochemistry of the Amazon basin, M. E. McClain, R. L. Victoria, J. E. Richey, Eds. (Oxford University Press, New York, 2001), pp. 165-184.
43. C. Palm et al., Environment, Development and Sustainability 6, 145 (2004).